The NIH Guidelines covers a specific subset of potentially biohazardous materials involving recombinant and/or synthetic nucleic acid molecules.
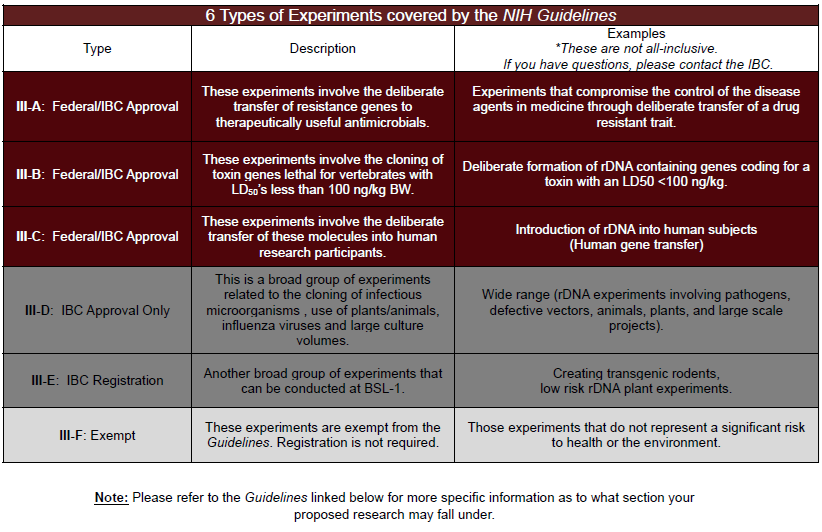
There are 6 types of experiments covered by the Guidelines. Each requires increasing levels of oversight by the institution and federal government.
Standards & Regulations
Incident Reporting
It is important to always report any laboratory incidents or exposures to the EH&S Biological Safety Officer and/or the IBC Chair. Incident reporting serves two primary goals:
- Determine the underlying root cause(s) in order to prevent opportunities for future recurrence.
- Maintain federal compliance since certain accidents or incidents involving recombinant or synthetic nucleic acids molecules must be reported to the NIH.
Incidents that require immediate (within 24 hours) reporting:
Spills and accidents which result in overt exposures to organisms containing recombinant or synthetic nucleic acid molecules must be immediately reported to the EH&S Biological Safety Officer, Institutional Biosafety Committee, and NIH OSP.
Incidents that must be reported within 30 days to the NIH OSP via the IBC:
- Any significant problems.
- Any violation of the NIH Guidelines.
- Any significant research-related accident or illness.